Innovative rapid microbial testing for the pharmaceutical industry
We have developed an innovative membrane filtration workflow for microbial testing applications for the pharmaceutical industry. The rapid microbiological method offers faster TTR combined with an easy workflow while providing all advantages of the traditional workflow. This allows faster product release and corrective actions during the full manufacturing process.
Through this questionnaire we would like to validate your overall interest in this workflow and the 2 possible loading solutions.
Through this questionnaire we would like to validate your overall interest in this workflow and the 2 possible loading solutions.

Issue
Solution
Context
Rapid Microbiological Methods have been of interest for the pharmaceutical industry for the last decade with a limited adoption rate due to high manual workload, need for highly skilled personnel, limited performances or difficulties to validate.
Solutions
We have developed an innovative membrane filtration solution for microbial testing (for example for water testing, bioburden, rapid screening) which provide faster results without changing the traditional workflow.
The workflow is very close to the traditional bioburden & pharmaceutical water testing workflow, with shorter TTR:
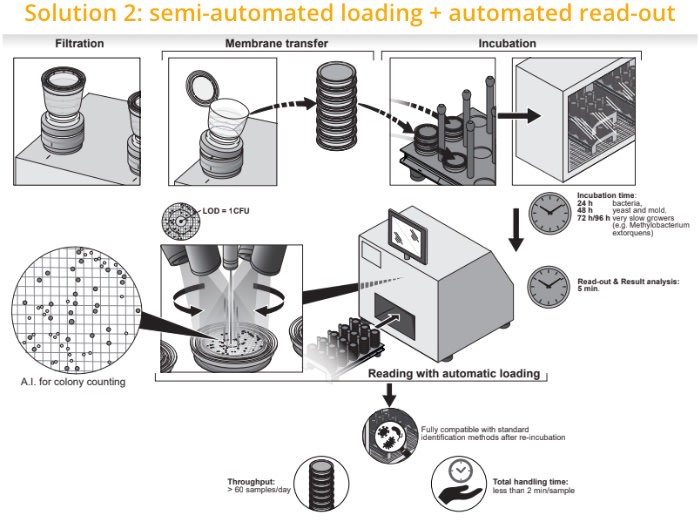
1. Sample filtration & a touch-free membrane transfer to the media plates
2. Incubation for 24h (bacteria), 48 hours (yeast & mold) or 72/96h for very slow growers (e.g. M.extorquens)
3. The loading can be done in 2 different ways:
The method will be validated in compliance with international standards and a service offer to support method implementation and validation by the supplier will be available.
Rapid Microbiological Methods have been of interest for the pharmaceutical industry for the last decade with a limited adoption rate due to high manual workload, need for highly skilled personnel, limited performances or difficulties to validate.
Solutions
We have developed an innovative membrane filtration solution for microbial testing (for example for water testing, bioburden, rapid screening) which provide faster results without changing the traditional workflow.
The workflow is very close to the traditional bioburden & pharmaceutical water testing workflow, with shorter TTR:
1. Sample filtration & a touch-free membrane transfer to the media plates
2. Incubation for 24h (bacteria), 48 hours (yeast & mold) or 72/96h for very slow growers (e.g. M.extorquens)
3. The loading can be done in 2 different ways:
- Manual loading (solution 1)
- Semi-automated loading (solution 2) in case of higher throughput need. It provides a charger to enable the loading of multiple samples which are automatically processed by the reader
The method will be validated in compliance with international standards and a service offer to support method implementation and validation by the supplier will be available.